INTERSTITIAL
From FEFF
Advanced
INTERSTITIAL inters totvol
The construction of interstitial potential and density may be changed by using this card. inters = ipot + 2*irav + 6*irmt. ipot=1 might be useful when only the surroundings of the absorbing atom are specified in ‘feff.inp’. irav and irmt are described only for completeness and nonzero values are strongly not recommended.
- ipot
- potential index. ipot defines how to find the interstitial potential: ipot=0 (default): the interstitial potential is found by averaging over the entire extended cluster in ‘feff.inp’. ipot=1 : the interstitial potential is found locally around the absorbing atom.
- irav
- also changes how interstitial potential is found. irav=0 (default): equation for Vint is constructed at rav=r_nrm. 1 : at rav=(r_mt+r_nrm)/2 , 2 - at rav=r_mt
- irmt
- irmt=0 (default): Norman prescription for mt radii. irav=1 : Matching point prescription for mt radii (do not use)
- totvol
- is the volume per atom normalized by ratmin3 (totvol=(volume per atom)/ratmin3), where ratmin is the shortest bond for the absorbing atom. This quantity defines the total volume (needed to calculate interstitial density) of the extended cluster specified in ‘feff.inp’. If totvol
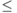 0 then the total volume is calculated as a sum of norman sphere volumes. Otherwise, totalvolume = nat * (vtot * ratmin3); where nat is a number of atoms in an extended cluster. Thus totvol=1.0 is appropriate for cubic structures, such as NaCl. The INTERSTITIAL card may be useful for open systems (e.g. those which have ZnS structure.
0 then the total volume is calculated as a sum of norman sphere volumes. Otherwise, totalvolume = nat * (vtot * ratmin3); where nat is a number of atoms in an extended cluster. Thus totvol=1.0 is appropriate for cubic structures, such as NaCl. The INTERSTITIAL card may be useful for open systems (e.g. those which have ZnS structure.
* improve interstitial density for ZnS structures. * totvol = (unit_cell_volume/number_of_atoms_in_unit_cell)/ratmin**3)=1.54 INTERSTITIAL 0 1.54
